Which Of The Following Is Thought To Be The Most Closely Related To The Common Animal Ancestor?
Abstract
Since the outbreak of severe acute respiratory syndrome (SARS) 18 years ago, a big number of SARS-related coronaviruses (SARSr-CoVs) accept been discovered in their natural reservoir host, bats1,ii,three,4. Previous studies accept shown that some bat SARSr-CoVs accept the potential to infect humansv,6,seven. Here we report the identification and characterization of a new coronavirus (2019-nCoV), which acquired an epidemic of acute respiratory syndrome in humans in Wuhan, Mainland china. The epidemic, which started on 12 Dec 2019, had caused 2,794 laboratory-confirmed infections including 80 deaths by 26 January 2020. Full-length genome sequences were obtained from five patients at an early stage of the outbreak. The sequences are almost identical and share 79.vi% sequence identity to SARS-CoV. Furthermore, we show that 2019-nCoV is 96% identical at the whole-genome level to a bat coronavirus. Pairwise protein sequence assay of seven conserved non-structural proteins domains show that this virus belongs to the species of SARSr-CoV. In addition, 2019-nCoV virus isolated from the bronchoalveolar lavage fluid of a critically ill patient could be neutralized by sera from several patients. Notably, we confirmed that 2019-nCoV uses the same cell entry receptor—angiotensin converting enzyme II (ACE2)—as SARS-CoV.
Master
Coronaviruses take acquired ii large-scale pandemics in the by ii decades, SARS and Middle Due east respiratory syndrome (MERS)8,9. Information technology has generally been idea that SARSr-CoV—which is mainly establish in bats—could crusade a future affliction outbreak10,11. Here we study on a series of cases acquired past an unidentified pneumonia illness outbreak in Wuhan, Hubei province, cardinal China. This disease outbreak—which started from a local seafood market place—has grown essentially to infect ii,761 people in China, is associated with eighty deaths and has led to the infection of 33 people in 10 additional countries as of 26 January 202012. Typical clinical symptoms of these patients are fever, dry cough, breathing difficulties (dyspnoea), headache and pneumonia. Disease onset may result in progressive respiratory failure owing to alveolar impairment (as observed by transverse chest computerized-tomography images) and even death. The disease was determined to exist caused by virus-induced pneumonia by clinicians according to clinical symptoms and other criteria, including a rise in trunk temperature, decreases in the number of lymphocytes and white blood cells (although levels of the latter were sometimes normal), new pulmonary infiltrates on chest radiography and no obvious comeback afterwards treatment with antibiotics for three days. Information technology appears that nearly of the early cases had contact history with the original seafood market; yet, the disease has at present progressed to be transmitted past human-to-human contact.
Samples from seven patients with severe pneumonia (half dozen of whom are sellers or deliverymen from the seafood market), who were admitted to the intensive care unit of measurement of Wuhan Jin Yin-Tan Hospital at the beginning of the outbreak, were sent to the laboratory at the Wuhan Institute of Virology (WIV) for the diagnosis of the causative pathogen (Extended Data Table ane). As a laboratory investigating CoV, nosotros showtime used pan-CoV PCR primers to test these samplesthirteen, given that the outbreak occurred in winter and in a market—the same surround as SARS infections. Nosotros found five samples to be PCR-positive for CoVs. One sample (WIV04), collected from the bronchoalveolar lavage fluid (BALF), was analysed by metagenomics analysis using next-generation sequencing to identify potential aetiological agents. Of the x,038,758 total reads—of which i,582 total reads were retained later filtering of reads from the man genome—1,378 (87.1%) sequences matched the sequence of SARSr-CoV (Fig. 1a). By de novo assembly and targeted PCR, we obtained a 29,891-base-pair CoV genome that shared 79.6% sequence identity to SARS-CoV BJ01 (GenBank accession number AY278488.2). High genome coverage was obtained by remapping the total reads to this genome (Extended Data Fig. 1). This sequence has been submitted to GISAID (https://www.gisaid.org/) (accession number EPI_ISL_402124). Following the name given by the World Health Organization (WHO), we tentatively call information technology novel coronavirus 2019 (2019-nCoV). Four more full-length genome sequences of 2019-nCoV (WIV02, WIV05, WIV06 and WIV07) (GISAID accession numbers EPI_ISL_402127–402130) that were more than 99.9% identical to each other were afterwards obtained from 4 boosted patients using adjacent-generation sequencing and PCR (Extended Information Tabular array 2).
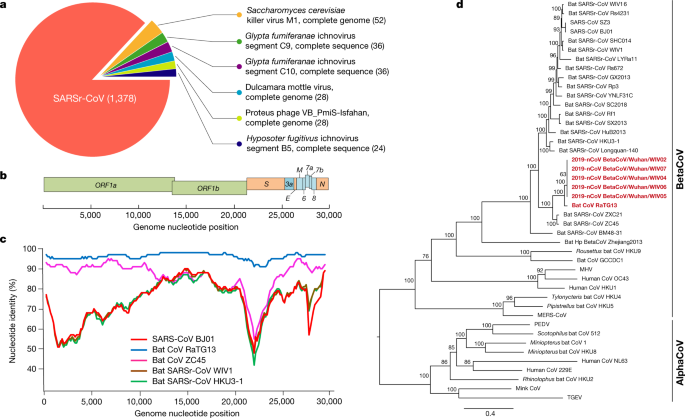
a, Metagenomics analysis of next-generation sequencing of BALF from patient ICU06. b, Genomic arrangement of 2019-nCoV WIV04. M, membrane. c, Similarity plot based on the full-length genome sequence of 2019-nCoV WIV04. Total-length genome sequences of SARS-CoV BJ01, bat SARSr-CoV WIV1, bat coronavirus RaTG13 and ZC45 were used as reference sequences. d, Phylogenetic tree based on nucleotide sequences of consummate genomes of coronaviruses. MHV, murine hepatitis virus; PEDV, porcine epidemic diarrhoea virus; TGEV, porcine transmissible gastroenteritis virus.The scale confined represent 0.1 substitutions per nucleotide position. Descriptions of the settings and software that was used are included in the Methods.
The virus genome consists of six major open up-reading frames (ORFs) that are mutual to coronaviruses and a number of other accessory genes (Fig. 1b). Further analysis indicates that some of the 2019-nCoV genes shared less than 80% nucleotide sequence identity to SARS-CoV. However, the amino acid sequences of the seven conserved replicase domains in ORF1ab that were used for CoV species classification were 94.4% identical between 2019-nCoV and SARS-CoV, suggesting that the two viruses belong to the same species, SARSr-CoV.
We then establish that a short region of RNA-dependent RNA polymerase (RdRp) from a bat coronavirus (BatCoV RaTG13)—which was previously detected in Rhinolophus affinis from Yunnan province—showed loftier sequence identity to 2019-nCoV. Nosotros carried out full-length sequencing on this RNA sample (GISAID accretion number EPI_ISL_402131). Simplot analysis showed that 2019-nCoV was highly similar throughout the genome to RaTG13 (Fig. 1c), with an overall genome sequence identity of 96.2%. Using the aligned genome sequences of 2019-nCoV, RaTG13, SARS-CoV and previously reported bat SARSr-CoVs, no show for recombination events was detected in the genome of 2019-nCoV. Phylogenetic analysis of the total-length genome and the cistron sequences of RdRp and spike (Due south) showed that—for all sequences—RaTG13 is the closest relative of 2019-nCoV and they form a distinct lineage from other SARSr-CoVs (Fig. 1d and Extended Information Fig. 2). The receptor-binding spike protein encoded by the S cistron was highly divergent from other CoVs (Extended Data Fig. ii), with less than 75% nucleotide sequence identity to all previously described SARSr-CoVs, except for a 93.1% nucleotide identity to RaTG13 (Extended Data Table 3). The South genes of 2019-nCoV and RaTG13 are longer than other SARSr-CoVs. The major differences in the sequence of the S cistron of 2019-nCoV are the three short insertions in the N-last domain as well as changes in four out of five of the key residues in the receptor-binding motif compared with the sequence of SARS-CoV (Extended Data Fig. 3). Whether the insertions in the N-last domain of the S protein of 2019-nCoV confer sialic-acid-binding activity every bit it does in MERS-CoV needs to be further studied. The close phylogenetic relationship to RaTG13 provides evidence that 2019-nCoV may have originated in bats.
We rapidly developed a qPCR-based detection method on the footing of the sequence of the receptor-bounden domain of the S gene, which was the most variable region of the genome (Fig. 1c). Our information evidence that the primers could differentiate 2019-nCoV from all other human coronaviruses including bat SARSr-CoV WIV1, which shares 95% identity with SARS-CoV (Extended Information Fig. 4a, b). Of the samples obtained from the seven patients, we found that six BALF and five oral swab samples were positive for 2019-nCoV during the outset sampling, as assessed by qPCR and conventional PCR. However, nosotros could no longer detect virus-positive samples in oral swabs, anal swabs and claret samples taken from these patients during the second sampling (Fig. 2a). However, we recommend that other qPCR targets, including the RdRp or envelope (Due east) genes are used for the routine detection of 2019-nCoV. On the basis of these findings, we propose that the disease could exist transmitted past airborne transmission, although nosotros cannot rule out other possible routes of transmission, as further investigation, including more patients, is required.
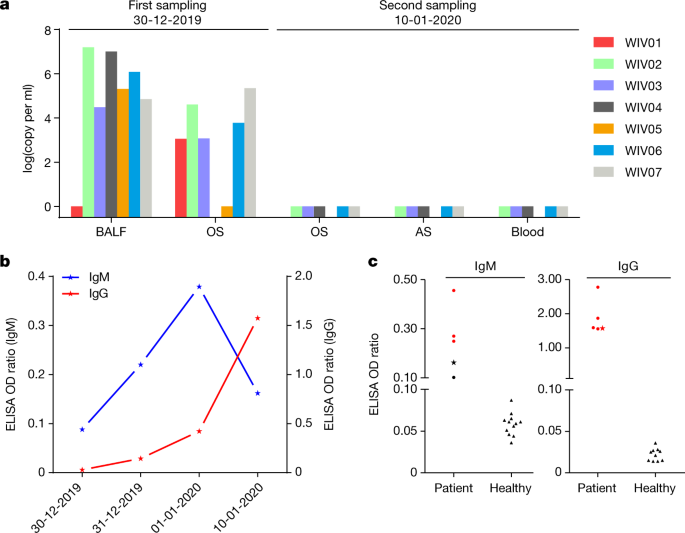
a, Molecular detection of 2019-nCoV in seven patients. Patient data can be found in Extended Information Tables 1, 2. Detection methods are described in the Methods. Equally, anal swab; OS, oral swab. b, Dynamics of 2019-nCoV antibody levels in one patient who showed signs of disease on 23 December 2019 (ICU-06). OD ratio, optical density at 450–630 nm. The right and left y axes indicate ELISA OD ratios for IgM and IgG, respectively. c, Serological exam of 2019-nCoV antibodies in five patients (Extended Information Tabular array two). The asterisk indicates information collected from patient ICU-06 on 10 January 2020. b, c, The cut-off was to 0.2 for the IgM analysis and to 0.3 for the IgG analysis, according to the levels of good for you controls.
For serological detection of 2019-nCoV, nosotros used a previously developed nucleocapsid (Northward) poly peptide from bat SARSr-CoV Rp3 every bit antigen for IgG and IgM enzyme-linked immunosorbent assays (ELISAs), equally this protein shared 92% amino acid identity to N protein of 2019-nCoV (Extended Information Fig. five) and showed no cross-reactivity against other human coronaviruses except SARSr-CoVvii. We were only able to obtain five serum samples from the 7 patients with viral infections. We monitored viral antibody levels in one patient (ICU-06) 7, viii, ix and 18 days later on the onset of illness (Extended Data Table ii). A clear trend was observed in the IgG and IgM titres, which increased over time, except that the IgM titre was decreased in the concluding sample (Fig. 2b). As a second analysis, we tested samples from 5 of the 7 virus-positive patients around 20 days after affliction onset for the presence of viral antibodies (Extended Data Tables 1, ii). All patient samples—merely not samples from healthy individuals—were strongly positive for viral IgG (Fig. 2b). There were also three IgM-positive samples, indicating an acute infection.
We next successfully isolated the virus (called 2019-nCoV BetaCoV/Wuhan/WIV04/2019) from both Vero E6 and Huh7 cells using the BALF sample of patient ICU-06. Clear cytopathogenic effects were observed in cells after incubation for three days (Extended Data Fig. 6a, b). The identity of the strain WIV04 was verified in Vero E6 cells by immunofluorescence microscopy using the cantankerous-reactive viral N antibiotic (Extended Data Fig. 6c, d) and by metagenomics sequencing, almost of the reads of which mapped to 2019-nCoV, and qPCR analysis showed that the viral load increased from solar day 1 to twenty-four hour period iii (Extended Data Fig. 6e, f). Viral particles in ultrathin sections of infected cells displayed a typical coronavirus morphology, every bit visualized by electron microscopy (Extended Information Fig. 6g). To further confirm the neutralization action of the viral IgG-positive samples, we conducted serum-neutralization assays in Vero E6 cells using the five patient sera that were IgG-positive. We demonstrate that all samples were able to neutralize 100 TCIDl (50% tissue-culture-infective dose) of 2019-nCoV at a dilution of i:40–1:eighty. We also show that this virus could exist cross-neutralized by horse anti-SARS-CoV serum (gift from L.-F. Wang) at dilutions of ane:40; however, the potential for cross-reactivity with SARS-CoV antibodies needs to be confirmed with anti-SARS-CoV serum from humans (Extended Data Table 4).
ACE2 is known to be a cell receptor for SARS-CoV14. To decide whether 2019-nCoV as well uses ACE2 every bit a cellular entry receptor, we conducted virus infectivity studies using HeLa cells that expressed or did not express ACE2 proteins from humans, Chinese horseshoe bats, civets, pigs and mice. Nosotros bear witness that 2019-nCoV is able to use all ACE2 proteins, except for mouse ACE2, as an entry receptor to enter ACE2-expressing cells, but not cells that did not express ACE2, indicating that ACE2 is probably the prison cell receptor through which 2019-nCoV enters cells (Fig. 3). We too evidence that 2019-nCoV does non use other coronavirus receptors, such every bit aminopeptidase N (APN) and dipeptidyl peptidase 4 (DPP4) (Extended Data Fig. 7).
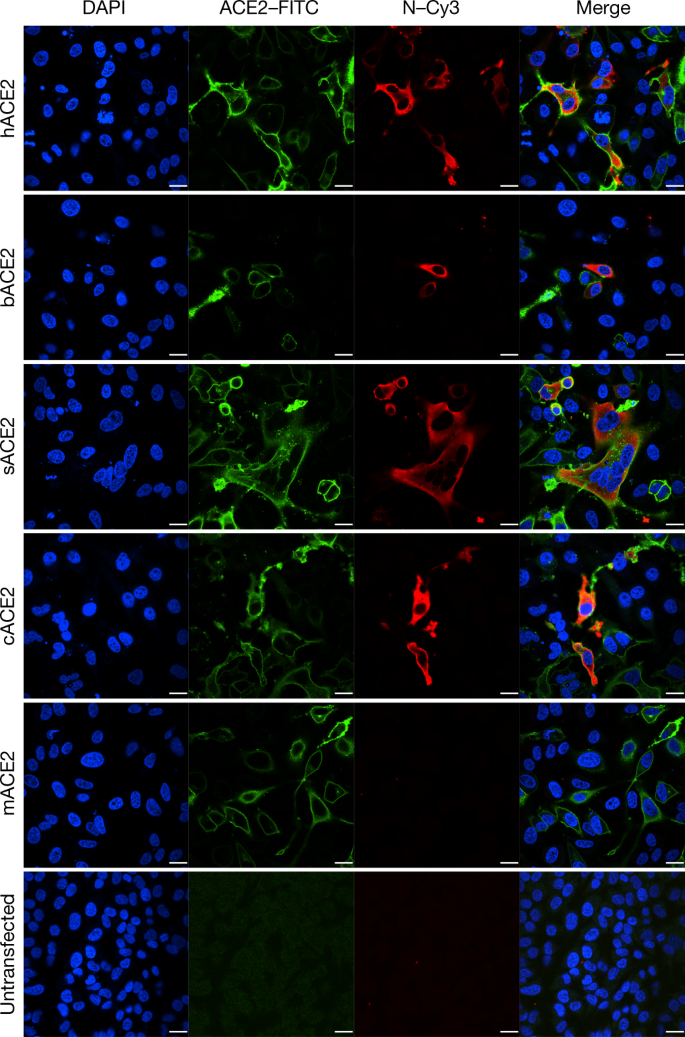
Determination of virus infectivity in HeLa cells that expressed or did non express (untransfected) ACE2. The expression of ACE2 plasmid with S tag was detected using mouse anti-S tag monoclonal antibiotic. hACE2, human ACE2; bACE2, ACE2 of Rhinolophus sinicus (bat); cACE2, civet ACE2; sACE2, swine ACE2 (grunter); mACE2, mouse ACE2. Green, ACE2; red, viral poly peptide (N); bluish, DAPI (nuclei). Scale confined, 10 μm.
The report provides a detailed study on 2019-nCoV, the likely aetiological agent responsible for the ongoing epidemic of acute respiratory syndrome in China and other countries. Virus-specific nucleotide-positive and viral-protein seroconversion was observed in all patients tested and provides evidence of an association between the disease and the presence of this virus. All the same, there are withal many urgent questions that remain to be answered. The clan betwixt 2019-nCoV and the affliction has non been verified by animate being experiments to fulfil the Koch's postulates to institute a causative relationship betwixt a microorganism and a disease. Nosotros do non still know the transmission routine of this virus among hosts. It appears that the virus is becoming more than transmissible between humans. We should closely monitor whether the virus continues to evolve to become more virulent. Owing to a shortage of specific treatments and because the relatedness of 2019-nCoV to SARS-CoV, some drugs and pre-clinical vaccines against SARS-CoV could probably be used to treat this virus. Finally, because the broad spread of SARSr-CoV in their natural reservoirs, future enquiry should exist focused on active surveillance of these viruses for broader geographical regions. In the long term, broad-spectrum antiviral drugs and vaccines should be prepared for emerging infectious diseases that are acquired by this cluster of viruses in the hereafter. Most importantly, strict regulations against the domestication and consumption of wild animals should be implemented.
Annotation added in proof: Since this paper was accepted, the ICTV has designated the virus as SARS-CoV-ii15; in addition, the WHO has released the official name of the disease caused past this virus, which is COVID-xix16.
Methods
Data reporting
No statistical methods were used to predetermine sample size. The experiments were not randomized and the investigators were not blinded to allocation during experiments and outcome cess.
Sample drove
Human samples, including oral swabs, anal swabs, claret and BALF samples were collected by Jinyintan hospital (Wuhan, China) with the consent of all patients and approved past the ethics commission of the designated infirmary for emerging infectious diseases. Patients were sampled without gender or historic period preference unless indicated. For swabs, 1.5 ml DMEM containing ii% FBS was added to each tube. The supernatant was nerveless later centrifugation at 2,500 rpm, vortexing for 60 south and a standing period of 15–30 min. The supernatant from swabs or BALF (no pre-treatment) was added to either lysis buffer for RNA extraction or to viral ship medium for isolation of the virus. The viral transport medium was composed of Hank's balanced salt solution (pH 7.4) containing BSA (1%), amphotericin (xv μg ml−1), penicillin M (100 units ml−1) and streptomycin (50 μg ml−1). Serum was separated by centrifugation at 3,000g for 15 min inside 24 h of collection, followed by inactivation at 56 °C for ane h, and was then stored at 4 °C until utilise.
Virus isolation, cell infection, electron microscopy and neutralization analysis
The post-obit jail cell lines were used for virus isolation in this study: Vero E6 and Huh7 cells, which were cultured in DMEM containing x% FBS. All cell lines were tested and complimentary of mycoplasma contagion, submitted for species identification and authenticated past morphological evaluation by microscopy. None of the jail cell lines was on the listing of normally misidentified jail cell lines (by ICLAC).
Cultured cell monolayers were maintained in their corresponding medium. The PCR-positive BALF sample from ICU-06 patient was spun at 8,000chiliad for 15 min, filtered and diluted one:2 with DMEM supplemented with 16 μg ml−1 trypsin before it was added to the cells. Subsequently incubation at 37 °C for one h, the inoculum was removed and replaced with fresh civilization medium containing antibiotics (encounter below) and 16 μg ml−1 trypsin. The cells were incubated at 37 °C and observed daily for cytopathogenic effects. The culture supernatant was examined for the presence of virus by qRT–PCR methods developed in this study, and cells were examined by immunofluorescence microscopy using the anti-SARSr-CoV Rp3 N antibody that was generated in-house (1:1,000). Penicillin (100 units ml−i) and streptomycin (15 μg ml−1) were included in all tissue civilization media.
Vero E6 cells were infected with the new virus at a multiplicity of infection (MOI) of 0.5 and collected 48 h afterward infection. Cells were fixed with 2.v% (w/v) glutaraldehyde and ane% osmium tetroxide, dehydrated through a graded serial of ethanol concentrations (from thirty to 100%) and embedded with epoxy resin. Ultrathin sections (80 nm) of embedded cells were prepared, deposited onto Formvar-coated copper grids (200 mesh), stained with uranyl acetate and lead citrate, and analysed using a 200-kV Tecnai G2 electron microscope.
The virus neutralization exam was carried out in a 96-well plate. The patient serum samples were heat-inactivated by incubation at 56 °C for i h earlier use. The serum samples were diluted to i:ten, 1:20, 1:40 or 1:fourscore, and then an equal book of virus stock was added and incubated at 37 °C for 60 min in a 5% CO2 incubator. Diluted horse anti-SARS-CoV serum or serum samples from salubrious individuals were used as control. Later on incubation, 100 μl mixtures were inoculated onto a monolayer of Vero E6 cells in a 96-well plate for one h. Each serum was assessed in triplicate. After removing the supernatant, the plate was washed twice with DMEM medium. Cells were incubated with DMEM supplemented with 2% FBS for three days. Subsequently, the cells were checked for cytopathogenic furnishings.
RNA extraction and PCR
Whenever commercial kits were used, the manufacturer'due south instructions were followed without modification. RNA was extracted from 200 μl of samples with the Loftier Pure Viral RNA kit (Roche). RNA was eluted in 50 μl of elution buffer and used as the template for RT–PCR.
For qPCR analysis, primers based on the Southward gene of 2019-nCoV were designed: RBD-qF1, 5′-CAATGGTTTAACAGGCACAGG-three′; RBD-qR1, 5′-CTCAAGTGTCTGTGGATCACG-3′. RNA extracted equally described to a higher place was used for qPCR using the HiScript II 1 Step qRT–PCR SYBR Green Kit (Vazyme Biotech). Conventional PCRs were also performed using the following primer pairs: ND-CoVs-951F, 5′-TGTKAGRTTYCCTAAYATTAC-3′; ND-CoVs-1805R, v′-ACATCYTGATANARAACAGC-3′. The 20-μl qPCR reaction mix contained 10 μl 2× One Step SYBR Dark-green mix, 1 μl One Step SYBR Greenish Enzyme mix, 0.iv μl 50× ROX Reference Dye 1, 0.4 μl of each primer (10 μM) and 2 μl template RNA. Amplification was performed as follows: 50 °C for 3 min, 95 °C for thirty due south followed past twoscore cycles consisting of 95 °C for ten s and 60 °C for xxx s, and a default melting curve stride in an ABI 7500 Real-fourth dimension PCR machine.
Serological test
In-house anti-SARSr-CoV IgG and IgM ELISA kits were adult using SARSr-CoV Rp3 N protein equally antigen, which shared more than than 90% amino acrid identity to all SARSr-CoVs2. For IgG analyses, MaxiSorp Nunc-immuno 96-well ELISA plates were coated (100 ng per well) overnight with recombinant N protein. Human sera were used at a dilution of i:20 for ane h at 37 °C. An anti-human IgG HRP-conjugated monoclonal antibody (Kyab Biotech) was used at a dilution of 1:40,000. The OD value (450–630 nm) was calculated. For IgM analyses, MaxiSorp Nunc-immuno 96-well ELISA plates were coated (500 ng per well) overnight with anti-human IgM (μ chain). Human sera were used at a 1:100 dilution for 40 min at 37 °C, followed past incubation with an anti-Rp3 N HRP-conjugated antibody (Kyab Biotech) at a dilution of ane:4,000. The OD value (450–630 nm) was calculated.
Exam of ACE2 receptor for 2019-nCoV infection
HeLa cells transiently expressing ACE2 were prepared using Lipofectamine 3000 (Thermo Fisher Scientific) in a 96-well plate; mock-transfected cells were used as controls. 2019-nCoV grown in Vero E6 cells was used for infection at a MOI of 0.five. APN and DPP4 were analysed in the same way. The inoculum was removed after absorption for 1 h and washed twice with PBS and supplemented with medium. At 24 h after infection, cells were washed with PBS and fixed with four% formaldehyde in PBS (pH 7.4) for 20 min at room temperature. ACE2 expression was detected using a mouse anti-Southward tag monoclonal antibody and a FITC-labelled goat anti-mouse IgG H&L (Abcam, ab96879). Viral replication was detected using a rabbit antibiotic confronting the Rp3 N protein (generated in-house, 1:1,000) and a Cy3-conjugated caprine animal anti-rabbit IgG (1:200, Abcam, ab6939). Nuclei were stained with DAPI (Beyotime). Staining patterns were examined using confocal microscopy on a FV1200 microscope (Olympus).
Loftier-throughput sequencing, pathogen screening and genome assembly
Samples from patient BALF or from the supernatant of virus cultures were used for RNA extraction and next-generation sequencing (NGS) using BGI MGISEQ2000 and Illumina MiSeq 3000 sequencers. Metagenomic analysis was carried out mainly based on the bioinformatics platform MGmapper (PE_2.24 and SE_2.24). The raw NGS reads were first candy by Cutadapt (v.1.18) with minimum read length of 30 base pairs. BWA (five.0.7.12-r1039) was used to align reads to a local database with a filter hits parameter of 0.8 FMM ((match + mismatch)/read length ≥ fraction] value and minimum alignment score of 30. Parameters for post-processing of assigned reads were set to a minimum size normalized abundance of 0.01, minimum read count of 20 and were otherwise ready to default parameters. A local nucleic acid database for human and mammals was used to filter reads of host genomes before mapping reads to the virus database. The results of the metagenomic analysis were displayed as pie charts using Microsoft Office 2010. NGS reads were assembled into genomes using Geneious (5.eleven.0.iii) and MEGAHIT (five.1.2.9). PCR and Sanger sequencing was performed to fill gaps in the genome. 5′-rapid amplification of cDNA ends (RACE) was performed to determine the v′-end of the genomes using a SMARTer RACE v′/3′ kit (Takara). Genomes were annotated using the Clone Manager Professional Suite 8 (Sci-Ed Software).
Phylogenetic analysis
Routine sequence management and assay was carried out using DNAStar. The sequence alignment of complete genome sequences was performed using MAFFT (v.vii.307) with default parameters. The codon alignments of full-length Due south and RdRp factor sequences were converted from the corresponding protein alignments past PAL2NAL (v.14); the poly peptide alignments were created by Clustal Omega (v.i.2.4) using default parameters. Maximum likelihood phylogenetic trees were generated using RAxML (v.0.9.0) with GTR+M substitution model and ane,000 bootstrap replicates.
Reporting summary
Farther information on research design is available in the Nature Enquiry Reporting Summary linked to this newspaper.
Information availability
Sequence data that back up the findings of this report have been deposited in GISAID (https://world wide web.gisaid.org/) with accession numbers EPI_ISL_402124, EPI_ISL_402127–EPI_ISL_402130 and EPI_ISL_402131; GenBank with accession numbers MN996527–MN996532; National Genomics Data Center, Beijing Institute of Genomics, Chinese Academy of Sciences (https://bigd.big.ac.cn/databases?lang=en) with accession numbers SAMC133236–SAMC133240 and SAMC133252.
Modify history
-
28 September 2020
This article was amended to correct the Peer review information.
References
-
Li, W. et al. Bats are natural reservoirs of SARS-like coronaviruses. Scientific discipline 310, 676–679 (2005).
-
Ge, X.-Y. et al. Isolation and label of a bat SARS-similar coronavirus that uses the ACE2 receptor. Nature 503, 535–538 (2013).
-
Yang, L. et al. Novel SARS-like betacoronaviruses in bats, China, 2011. Emerg. Infect. Dis. 19, 989–991 (2013).
-
Hu, B. et al. Discovery of a rich gene pool of bat SARS-related coronaviruses provides new insights into the origin of SARS coronavirus. PLoS Pathog. 13, e1006698 (2017).
-
Menachery, Five. D. et al. A SARS-similar cluster of circulating bat coronaviruses shows potential for homo emergence. Nat. Med. 21, 1508–1513 (2015).
-
Menachery, V. D. et al. SARS-like WIV1-CoV poised for man emergence. Proc. Natl Acad. Sci. United states of america 113, 3048–3053 (2016).
-
Wang, N. et al. Serological evidence of bat SARS-related coronavirus infection in humans, China. Virol. Sin. 33, 104–107 (2018).
-
Drosten, C. et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 348, 1967–1976 (2003).
-
Zaki, A. One thousand., van Boheemen, S., Bestebroer, T. Grand., Osterhaus, A. D. G. E. & Fouchier, R. A. One thousand. Isolation of a novel coronavirus from a human being with pneumonia in Kingdom of saudi arabia. N. Engl. J. Med. 367, 1814–1820 (2012).
-
Cui, J., Li, F. & Shi, Z. L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 17, 181–192 (2019).
-
Fan, Y., Zhao, K., Shi, Z.-L. & Zhou, P. Bat coronaviruses in Red china. Viruses 11, 210 (2019).
-
Wuhan Municipal Health Commission. Press statement related to novel coronavirus infection (in Chinese) http://wjw.wuhan.gov.cn/forepart/spider web/showDetail/2020012709194 (2020).
-
Poon, L. L. et al. Identification of a novel coronavirus in bats. J. Virol. 79, 2001–2009 (2005).
-
Li, W. et al. Angiotensin-converting enzyme ii is a functional receptor for the SARS coronavirus. Nature 426, 450–454 (2003).
-
Gorbalenya, A. E. et al. Severe astute respiratory syndrome-related coronavirus — the species and its viruses, a argument of the Coronavirus Written report Group. Preprint at https://www.biorxiv.org/content/10.1101/2020.02.07.937862v1 (2020).
-
WHO. WHO Director-General's remarks at the media briefing on 2019-nCoV on 11 Feb 2020. https://www.who.int/dg/speeches/item/who-director-general-s-remarks-at-the-media-conference-on-2019-ncov-on-11-february-2020 (WHO, eleven February 2020).
Acknowledgements
We thank P. Zhang and A. Du from the WIV core facility centre for their help with producing transmission electron microscopy micrographs; H.-Z. Liu and P. Yu from WIV for bioinformatics assay. This work was jointly supported by the Strategic Priority Inquiry Programme of the Chinese Academy of Sciences (CAS) (XDB29010101 to Z.-L.S. and XDB29010104 to P.Z.), Prc Natural Science Foundation for excellent scholars (81822028 to P.Z., 31770175 to Z.-50.South. and 31800142 to B.H.), Mega-Project for Infectious Disease from Minister of Science and Technology of the People's Republic of China (2018ZX10305409-004-001 to P.Z.), Youth innovation promotion association of CAS (2019328 to X.-L.Y.).
Author information
Affiliations
Contributions
Z.-L.S., P.Z., Y.-Y.W. and 1000.-F.Ten. conceived the report. X.-G.W., C.-L.H., H.-D.C., F.D., Q.-J.C., F.-X.Z. and L.-Fifty.Fifty. collected patient samples. X.-Fifty.Y., B.Y., W.Z., B.L., J.C., 10.-S.Z., Y.L., H.G., R.-D.J., K.-Q.L., Y.C., X.W., X.-R.Southward. and K.Z. performed qPCR, serology and virus culturing experiments. L.Z., Y.Z., H.-R.Due south. and B.H. performed genome sequencing and annotations.
Respective author
Ethics declarations
Competing interests
The authors declare no competing interests.
Boosted information
Peer review information Nature cheers the anonymous reviewers for their contribution to the peer review of this piece of work.
Publisher's notation Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended information figures and tables
Extended Data Fig. 1 NGS raw reads of sample WIV04 mapping to the 2019-nCoV sequence.
The ten axis indicates the genome nucleotide position and the y axis represents the read depth of the mapping.
Extended Data Fig. 2 Phylogenetic trees based on the complete South and RdRp factor sequences of coronaviruses.
a, b, Phylogenetic trees on the basis of the gene sequences of S (a) and RdRp (b) are shown. 2019-nCoV and bat CoV RaTG13 are shown in assuming and in red. The trees were constructed using the maximum likelihood method using the GTR + Thou commutation model with bootstrap values determined by 1,000 replicates. Bootstraps values of more than than 50% are shown.
Extended Data Fig. 3 Amino acrid sequence alignment of the S1 protein of the 2019-nCoV to SARS-CoV and selected bat SARSr-CoVs.
The receptor-binding motif of SARS-CoV and the homologous region of other coronaviruses are indicated by the red box. The key amino acrid residues involved in the interaction with human ACE2 are numbered at the top of the aligned sequences. The short insertions in the N-terminal domain of the 2019-nCoV are indicated by the blue boxes. Bat CoV RaTG13 was obtained from R. affinis, institute in Yunnan province. Bat CoV ZC45 was obtained from R. sinicus, found in Zhejiang province.
Extended Information Fig. iv Molecular detection method used to detect 2019-nCoV.
a, Standard curve for qPCR primers. The PCR product of the S gene that was serial diluted in the range of 108 to 101 (lines from left to right) was used as a template. Primer sequences and experimental conditions are described in the Methods. b, Specificity of the qPCR primers. Nucleotide samples from the indicated pathogens were used.
Extended Data Fig. 6 Isolation and antigenic characterization of 2019-nCoV.
a, b, Vero E6 cells are shown at 24 h after infection with mock virus (a) or 2019-nCoV (b). c, d, Mock-virus-infected (c) or 2019-nCoV-infected (d) samples were stained with rabbit serum raised against recombinant SARSr-CoV Rp3 Northward protein (cerise) and DAPI (blue). The experiment was conducted twice independently with like results. e, The ratio of the number of reads related to 2019-nCoV among the total number of virus-related reads in metagenomics analysis of supernatants from Vero E6 prison cell cultures. f, Virus growth in Vero E6 cells. g, Viral particles in the ultrathin sections were imaged using electron microscopy at 200 kV. The sample was from virus-infected Vero E6 cells. The inset shows the viral particles in an intra-cytosolic vacuole.
Extended Information Fig. vii Analysis of 2019-nCoV receptor usage.
Conclusion of virus infectivity in HeLa cells with or without the expression of man APN and DPP4. The expression of ACE2, APN and DPP4 plasmids with South tag were detected using mouse anti-Due south tag monoclonal antibody. ACE2, APN and DPP4 proteins (greenish), viral poly peptide (red) and nuclei (blue) are shown. Scale bars, ten μm.
Supplementary data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long equally you give appropriate credit to the original writer(s) and the source, provide a link to the Artistic Eatables license, and indicate if changes were fabricated. The images or other tertiary party cloth in this article are included in the article'southward Artistic Commons license, unless indicated otherwise in a credit line to the material. If material is non included in the article'southward Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you volition need to obtain permission directly from the copyright holder. To view a re-create of this license, visit http://creativecommons.org/licenses/past/4.0/.
Reprints and Permissions
Nigh this article
Cite this commodity
Zhou, P., Yang, XL., Wang, XG. et al. A pneumonia outbreak associated with a new coronavirus of likely bat origin. Nature 579, 270–273 (2020). https://doi.org/x.1038/s41586-020-2012-seven
-
Received:
-
Accepted:
-
Published:
-
Result Date:
-
DOI : https://doi.org/10.1038/s41586-020-2012-7
Further reading
-
Scavenger receptors in host defense: from functional aspects to manner of action
Cell Communication and Signaling (2022)
-
Telescopic of SARS-CoV-2 variants, mutations, and vaccine technologies
The Egyptian Journal of Internal Medicine (2022)
-
Monoclonal antibodies for COVID-xix therapy and SARS-CoV-2 detection
Journal of Biomedical Science (2022)
-
Programmable Biosensors Based on RNA-Guided CRISPR/Cas Endonuclease
Biological Procedures Online (2022)
-
Intelligent system based comparative assay report of SARS-CoV-2 spike protein and antigenic proteins in different types of vaccines
Beni-Suef University Periodical of Basic and Applied Sciences (2022)
Comments
By submitting a annotate y'all agree to abide past our Terms and Community Guidelines. If you detect something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.
Source: https://www.nature.com/articles/s41586-020-2012-7
Posted by: walstoncoulut.blogspot.com

0 Response to "Which Of The Following Is Thought To Be The Most Closely Related To The Common Animal Ancestor?"
Post a Comment